Author: Dylan Daniel, PhD, Director, Scientific Development
Date: August 2016
Multiple myeloma is a malignancy of plasma B cells and is the second most common hematological malignancy in the United States. Malignant myeloma cells accumulate in the bone marrow and ultimately replace normal hematopoetic stem cells, which results in progressive leukocyte deficiencies. Chemotherapeutic agents and proteasome inhibitors are standard-of-care front line therapy that is often followed by autologous stem cell transplantation. Relapse is common with these treatment strategies,1 and research toward novel targeted therapies and immuno-therapies is being pursued aggressively.
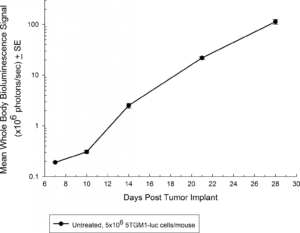
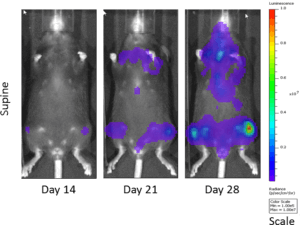
Fig. 1: Growth Kinetics of 5TGM1-luc in Immune-Competent C57BL/KaLwRij Mice by BLI
Fig. 2: Bioluminescent Imaging of 5TGM1 Cells in C57BL/KaLwRij Mice
The 5TGM1 multiple myeloma model arose spontaneously in aging C57BL/KaLwRij mice, and was propagated via serial passage in syngeneic mice prior to establishment of a cell line. Our 5TGM1 line has been luciferase-enabled to permit monitoring of growth in orthotopic sites such as the bone marrow by bioluminescent imaging (BLI). Intravenous implantation of 5TGM1-luc into syngeneic C57BL/KaLwRij mice results in progressive growth of the cells as monitored by BLI (Figure 1) with signal evident in the long bones (marrow) and other disseminated sites including lung, liver, spleen, spine, and brain (Figure 2). The 5TGM1-luc cells will also grow in an immune-deficient strain, the NIH-III Nude, aka beige nude xid, mouse with a more rapid time to evaluation of tumor burden in the bone.
Proteasome inhibitors have also become standard of care agents in the treatment of multiple myeloma. In the NIH-III mice, at the dose and regimen tested, the 5TGM1-luc model is completely non-responsive to carfilzomib (Figure 3a) by evaluation of whole body BLI; however, in the syngeneic setting, the 5TGM1-luc model shows a trend toward tumor growth delay (Figure 3b) that could be ideal for combination approaches.
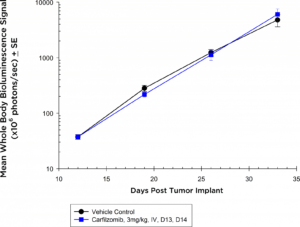
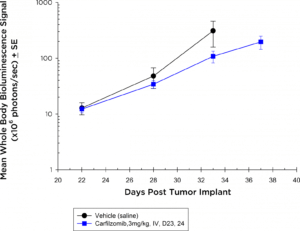
Fig. 3a: Anti-tumor Effects of Carfilzomib on Disseminated 5TGM1-luc Disease in NIH-III Nude Mice
Fig. 3b: Anti-tumor Effects of Carfilzomib on Disseminated 5TGM1-luc Disease in C57BL/KaLwRij Mice
In these studies while we did not observe significant activity with analysis of whole body tumor burden, the flexibility of BLI and/or ex vivo analysis of tumor burden in the bone marrow by flow cytometry could allow us to refine our analysis to specific anatomical sites in future studies that could reveal therapeutic benefits. Multiple clinical trials are ongoing, testing checkpoint inhibitors and other immuno-oncology agents in multiple myeloma. Anti-PD-12 and anti-CD1373 antibodies are active in the 5TGM1 model which suggests that it’s a valid model for testing novel immuno-oncology agents.
References
1Naymagon et al., J Hematol Oncol. (2016) 9: 52.
2Paiva et al., Leukemia (2015) 29: 2110–2113.
3Murillo et al., Clin. Cancer Res. (2008) 14: 6895-6906.
Connect
Let's start a conversation
Contact Us