Author: Dylan Daniel, PhD, Director, Scientific Development
Date: April 2017
Acute myelogenous leukemia (AML) is a rapidly progressing malignancy of myeloid white blood cells. An estimated 21,380 new cases of AML and 10,590 deaths will occur in the United States in 2017. AML results from excessive proliferation of dedifferentiated or undifferentiated myeloid leukocytes initially in the bone marrow. As disease progresses, AML blast cells will be found in the blood and other secondary lymphoid organs. Symptoms including anemia and leukopenia result from proliferating AML cells suppressing normal hematopoiesis in the bone marrow. AML is characterized by a complex number of subtypes that are defined by genomic alterations and gene expression profiling. Treatment typically starts with intensive chemotherapy followed by allogeneic bone marrow transplantation (BMT) in patients that can tolerate these therapies. Less debilitating treatment options, including immunotherapy, are needed for older AML patients who frequently cannot tolerate intensive chemotherapy or BMT.
C1498 is a murine AML cell line that arose spontaneously in a C57BL/6 mouse and grows aggressively in syngeneic mice. Our C1498 line has been luciferase- and mCherry-enabled to permit monitoring of whole body tumor growth by bioluminescence imaging (BLI) and quantification of tumor burden in isolated tissues by flow cytometry, respectively. Intravenous implantation of C1498-luc-mCherry into syngeneic C57BL/6 mice results in progressive growth of the cells as monitored by BLI (Figure 1A) with signal evident in the long bones (bone marrow) and other disseminated sites during disease progression (Figure 1B).
Growth Kinetics and Representative BLI Images
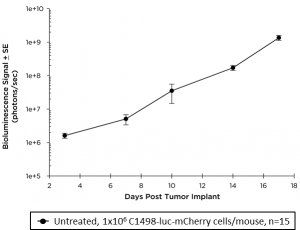
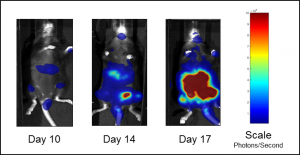
Fig. 1A: Growth kinetics of C1498-luc-mCherry in C57BL/6 Mice by BLI.
Fig. 1B: Representative BLI images of C1498-luc-mCherry tumor burden in untreated mice.
Survival (morbidity/mortality) kinetics (Figure 2A) show a median survival of approximately 23 days; although, the mice show no body weight loss during the course of disease progression (Figure 2B).
Survival Kinetics and Body Weight Change
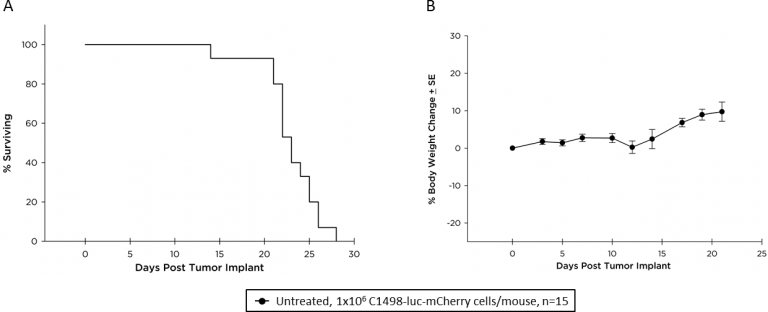
Fig. 2: Survival kinetics (A) and body weight change (B) of C57BL/6 mice with disseminated C1498-luc-mCherry AML.
Macroscopic tumor burden was evaluated at the time of necropsy (Table 1). The most prevalent sites for macroscopic tumors were liver, lymph nodes, ovaries and the spinal column. By utilizing mCherry expression in the C1498 line, tissue tumor burden can be analyzed by flow cytometry at the time of animal necropsy. A representative gating scheme to quantify mCherry+ AML cells from lymph nodes is shown in Figure 3. At day 21, AML tumor burden as a percent of total cells in lymph nodes, ovaries, and liver is extensive (Table 2).

Table 1: Incidence of macroscopic tumor lesions in tissues from mice with C1498-Luc-mCherry AML tumors.
Flow Cytometry Gating Strategy and Tissue Sampling
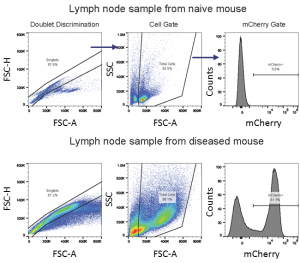
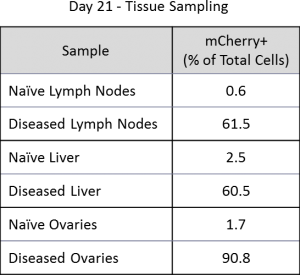
Fig. 3: Flow cytometry gating strategy for assessment of mCherry+ C1498 AML cells in a lymph node sample.
Table 2: Percentage of C1498-Luc-mCherry+ AML cells in tissues (n=1).
There are multiple ongoing clinical trials for immunotherapies in AML, including more than 15 trials with anti-PD-1 or anti-PD-L1 checkpoint inhibitors. C1498 has been reported to express low levels of PD-L1 in cell culture, but significantly upregulate PD-L1 expression in vivo.1 The expression of PD-L1 in vivo renders the model sensitive to tumor growth delay by PD-1 blockade. This is accompanied by a significant increase of CD4+ and CD8+ T cell infiltration in AML diseased liver. These published data suggest that C1498-luc-mCherry would be an ideal model to test immunotherapy combinations with checkpoint inhibitors.
Contact us to speak with one of our scientists to see how C1498-luc-mCherry or one of our other syngeneic models can be used for your next immuno-oncology study.
References
1Zhang, T. F. Gajewski and J. Kline, Blood, (2009).
Connect
Let's start a conversation
Contact Us