Author: Mark J Cameron, Director of Scientific Development
Date: November 2020
During preclinical research and development, CAR (chimeric antigen receptor) T-cell therapies are often investigated using a mouse as the test subject. In this setting, immunocompromised mice carry a tumor burden of human origin and are dosed with allogeneic human CAR T cells. The adoptively transferred cells are directed by the engineered chimeric antigen receptor to bind to a surface antigen on a tumor cell (CD19 for example).
As a result of the engineered T cell binding to the tumor cell, the T cell becomes activated and kills tumor cells in an MHC unrestricted manor. Mice in preclinical research studies are followed for a number of predefined parameters, evaluating tumor growth and burden to determine the efficacy of the CAR T cells (Learn more about how efficacy can be determined by bioluminescence imaging (BLI).
A key success factor of the CAR T cell antitumor response is how long the CAR T cells exist after infusion into the host (persistence). Despite full functionality, it has been shown that poor persistence of CAR T cells can limit an effective antitumor response.1 In the clinical setting, long-term remission in patients with hematological malignancies is associated with sustained persistence of CAR T cells.2 Co-stimulatory signaling is thought to influence T cell expansion and persistence. Preclinical studies investigating the efficacy of CAR constructs that are engineered to include the 4-1BB co-stimulator domain have been associated with long CAR T cell persistence, a slower and sustained effector function, and a higher proportion of memory T cells.3 Assessing CAR T persistence in preclinical studies have implications in determining clinical success.
This technology spotlight will provide the reader with information about measuring CAR T cell persistence, using flow cytometry during the preclinical phase.
Mouse Models for CAR T Cell Candidate Evaluation
In September of 2017 the Food and Drug Administration approved the first CAR T cell therapy. The cell therapy is marketed by Novartis and is called Kymriah. Kymriah is also known as tisagenlecleucel and was approved for children and young adults who no longer respond to standard therapies for B cell acute lymphoblastic leukemia. Kymriah is based on the work of Carl June, PhD., at the University of Pennsylvania in NSGTM mice.
The NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mouse, commonly known by the branded name, NOD scid gamma (NSG™), do not express the Prkdc gene nor the X-linked Il2rg gene. The scid (severe combined immune deficiency) mutation is in the DNA repair complex protein Prkdc and renders the mice B and T cell deficient. These mice do not have complement nor natural killer (NK) cells and are deficient in cytokine signaling pathways. These deficiencies make the mice ideal and highly amenable to engraftment of human immune cells, including CAR T cells. The mice engraft a wide range of human tumors and are the gold standard for preclinical CAR T cell research.
In our Preclinical Oncology department, we can use any commercially available mouse strain for CAR T cell therapy and other adoptive cell transfer studies, including NSGTM mice and, under certain conditions, we can accept specialty mice as well.
The PersistenceTTM Panel from Preclinical Oncology is used to generate longitudinal CAR T cell persistence data can be used with as little as 5 uL of whole blood. It is formatted as a lyse/no wash assay with absolute counts. The panel has flexibility, offering the opportunity to add specific anti-CAR probes in the FITC, PE and APC channels. NSG mice from the Jackson Laboratory (Bar Harbor, Maine, USA) were administered 2x107 human PBMCs, whole blood was drawn 7-, 14- and 21-days post administration and subjected to the PersistenceTTM Panel evaluation.
Table 1 describes the reagents used in the PersistenceTTM Panel. The gating strategy is detailed in Figure 1.
Antibody/Dye |
Description |
|---|---|
mCD45 |
Mouse pan-hematopoietic cells |
hCD45 |
Human pan-hematopoietic cells |
hCD3 |
Human pan-T cell marker |
hCD4 |
Human CD4+ T cell marker |
hCD8 |
Human CD8+ T cell marker |
Counting Beads |
Provides absolute counts |
Viability Dye |
Dead cell exclusion |
Optional: CAR Specific |
FITC, PE or APC |
Table 1: PersistenceTTM Panel of antibodies and description of their use.
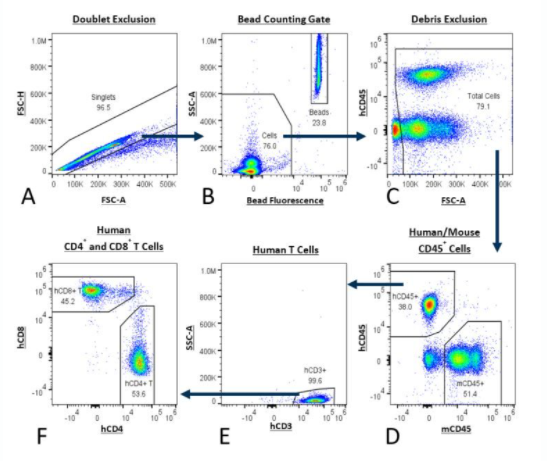
Figure 1: Analysis of human T cells using the PersistenceTTM Panel. The gating strategy begins with double exclusion (A) and moves to combining bead fluorescence with a bead counting gate (B). Next debris is excluded (C) and mouse cells are distinguished from human cells using anti-mouse CD45 with anti-human CD45 (D). Human T cells are isolated by anti-human CD3 gating (E) and finally the number of human CD8+ and CD4+ T cells are determined (F).
Longitudinal Immunophenotypic Characterization of CAR T Cells Using the PersistenceTTM Panel
In the clinical oncology setting, CAR T cell expansion and persistence correlates with response and attaining remission in patients.4 Because of this observation, establishing reliable methods of tracking CAR T cell numbers is particularly important, not only in the estimation of effectiveness of CAR T cell therapy, but in terms of safety evaluation in the preclinical setting. Serious side effects of clinical CAR T cell therapy are noted, one of the most severe being cytokine release syndrome (CRS) and is associated with CAR T cell expansion in vivo.5
The PersistenceTTM Panel is directly applicable to the evaluation of human CAR T cell numbers in samples taken from mice over time. Because of the low whole blood requirements, samples can be taken from the same mouse several times or by using cohorts of mice to accomplish study goals. NSG mice may be placed on long duration studies for focused CAR T persistence assessment. An example of how the PersistenceTTM Panel can be used to measure human T cells in NSG mice over time is shown in Figure 2.
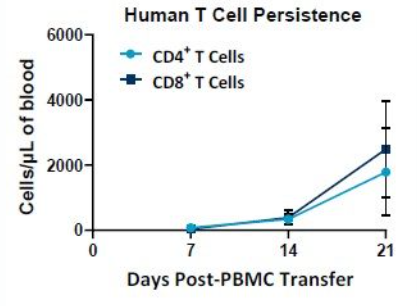
Improving CAR T Cell Persistence
In vivo CAR T cell persistence is a surrogate marker of long-term clinical efficacy of CAR T cell therapy. Studies have shown that certain CAR T cells, containing a CD28 costimulatory domain exhibit increased expression of T cell exhaustion-related genes, while the 4-1BB (TNFSF9) costimulatory domain with the same antigen specificity reduced the exhausted phenotype. This may help explain why in clinical trials of patients with relapsed or refractory Acute Lymphoblastic Leukemia, CAR T cells expressing a CD28 domain have been reported to persist for up to 3 months, while CAR T cells with a 4-1BB domain persist for up to 5 years, and more than 6 months in almost all of the cases that could be evaluated.6
CAR T Cells Through the Generations
CAR T cells continue to evolve through modification of the chimeric antigen receptor. Every CAR T cell has a scFv (single-chain fragment variables). The scFv is a combination of the variable region of heavy chain (VH) and variable region of light chain (VL) domains. An scFv is a fusion protein of VH and VL of immunoglobulins, connected with a short linker peptide of between 10 and 25 amino acids.
First generation CARs have an extracellular binding domain, a hinge region, a transmembrane domain and one or more intracellular signaling domains. All CAR T cells have the CD3 ζ chain domain for intracellular signaling and is the primary transmitter of T cell activation signals. The addition of a co-stimulatory domain (CD28 or 4-1BB) defined second generation CARs. The aim was to improve T cell proliferation, cytokine secretion and in vivo persistence.
Preclinical data shows that third generation CARs have improved effector function and longer in vivo persistence in contrast to second generation CARs. Third generation CARs have multiple co-stimulatory domains (CD28-41BB or CD28-OX40). Armored CARs and TRUCKs (CAR T cells redirected for universal cytokine killing) are names sometimes used for fourth generation CAR T cells. Fourth generation CAR T cells can include factors that enhance T cell expansion, anti-tumor activity and persistence.7
Regardless of the CAR or TRUCK generation, the PersistenceTTM Panel provides an excellent tool for short- or long-term monitoring of CAR T cell persistence in vivo in the preclinical setting.
Summary
Measuring CAR T persistence is made simple with the PersistenceTTM panel. Our flow cytometry panel allows for determination of longitudinal CAR T persistence from a small volume of blood. Our Human CompTTM Panel (Table 2) is an excellent example of how other qualified panels may be added to a preclinical CAR T study for generation of comprehensive sets of flow cytometry data.
Antibody/Dye |
Description |
|---|---|
hCD45 |
Human pan-hematopoietic cells |
hCD3 |
Human pan-T cell marker |
hCD4 |
Human CD4+ T cell marker |
hCD8 |
Human CD8+ T cell marker |
hCD25 |
Human regulatory T cell marker |
hFoxP3 |
Human regulatory T cell marker |
hPD-1 |
Human T cell inhibitory signaling protein |
hCD69 |
Activation marker |
Ki-67 |
Proliferation marker |
Viability Dye |
Dead cell exclusion |
Table 2. Individual markers in the Human CompTTM Leukocyte Panel.
To learn more about how the PersistenceTTM Panel, or one of our other flow cytometry panels, can be incorporated into your preclinical oncology research and to learn more about our extensive flow cytometry program contact the scientists.
References
1Song DG, Ye Q, Carpenito C, Poussin M, Wang LP, Ji C, Figini M, June CH, Coukos G, Powell DJ Jr. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB).
Cancer Res. 2011 Jul 1;71(13):4617-27.
2Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17.
3Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–90.
4Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139.
5Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The Other Side of CAR T-Cell Therapy: Cytokine Release Syndrome, Neurologic Toxicity, and Financial Burden.
Am Soc Clin Oncol Educ Book. 2019 Jan;39:433-444.
6Maus MV and June CH. Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy. Clin Cancer Res. 2016 Apr 15; 22(8): 1875–1884.
7Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15(8):1145-54.
Connect
Let's start a conversation
Contact Us