Date: Aug 2020
In order to provide a more reflective model of how tumor cells would respond to test agents in vivo, the tissue physiology where the tumor grows and proliferates needs to be recapitulated in vitro. This process has been accomplished using Predictive Oncology® organ-specific three-dimensional (3D) matrices that support long-term culture of carcinoma cells. Labcorp and Predictive Oncology® have worked together to develop tumor-specific 3D models, based on Predictive Oncology® proprietary 3D cell culture platform for preclinical testing and research studies in the biopharmaceutical industry. This tech spotlight highlights the characteristics of the Reconstructed Bone (r-Bone) model.
Growth of tumor cells within the bone marrow is a common phenomenon in hematologic malignancies. Thus, human myeloma cell lines RPMI 8226, NCI-H929, and U266B1 were utilized in proof-of-concept studies. As shown in Figure 1, U266B1 human myeloma cells were cultured in growth medium alone (2D) or in r-Bone matrix for 4 days. While the 2D culture becomes quickly overgrown and the cells maintain a morphology characterized by a single cell suspension, the r-Bone 3D culture supports the development of cell aggregates which proliferate into larger clusters more representative of tumor morphology. These cultures can be maintained for long periods with minor intervention by the researcher.
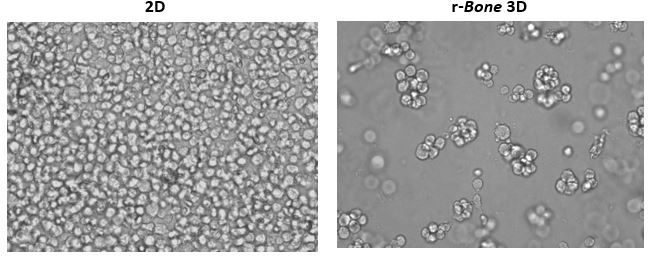
Figure 1: 2D vs Reconstructed Bone (r-Bone) 3D culture of U266B1 human myeloma cells
Figure 2 shows cryo-electron microscopic images of r-Bone culture and ex vivo bone marrow seeded with metastatic breast cancer cells. The spatial and physical architecture of bone marrow stroma is maintained in the r-Bone extracellular matrix (ECM). The r-Bone ECM allows for co-culture of tumor cells with mesenchymal stem cells, fibroblasts, lymphoid, myeloid, and CAR-T cells.
Cells can be isolated from the matrix after test agent exposure and analyzed by an appropriate downstream application, such as flow cytometry, RNASeq, or in situ imaging. Cytotoxicity assays such as CellTiter-Glo® can be performed in a high throughput format with the successful development of both 96 and 384-well assay formats. The platform is compatible with all drug classes tested, including biologics and small molecules.
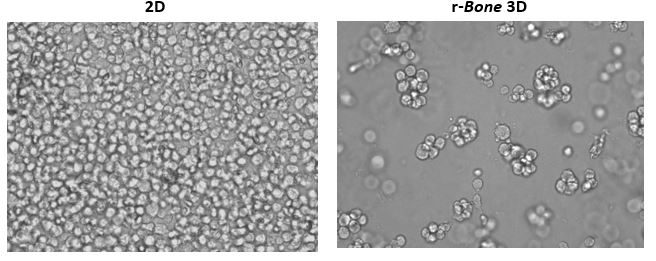
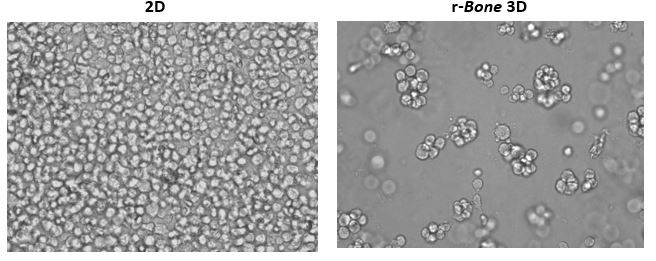
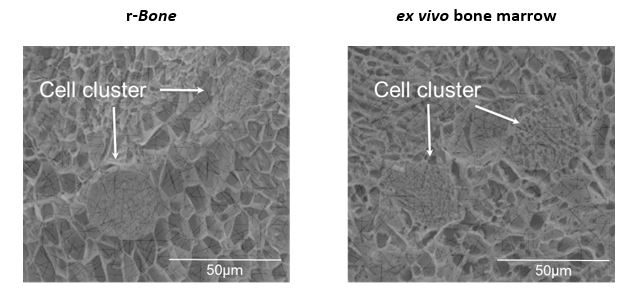
Figure 2: Extracellular matrix architecture of r-Bone is indistinguishable from ex vivo tissue
Figure 3: Bortezomib is a standard of care agent for the treatment of multiple myeloma2. After 4-day culture of NCI-H929, RPMI 8226, and U266B1 cells in r-Bone, bortezomib was added and the cells were returned to culture for an additional 4 days. (A) CellTiter-Glo® reagent was added and luminescence was quantified using a Cytation 3 imaging plate reader (Biotek Instruments). Four parameter nonlinear curve fitting analysis was normalized against the vehicle treated wells (n = 3 wells per concentration). (B) Representative images of U266B1 cells in r-Bone culture treated with vehicle (0.5% DMSO) or 20nM bortezomib. The red arrows indicate dead or dying cells in the cell clusters after 72hr exposure.
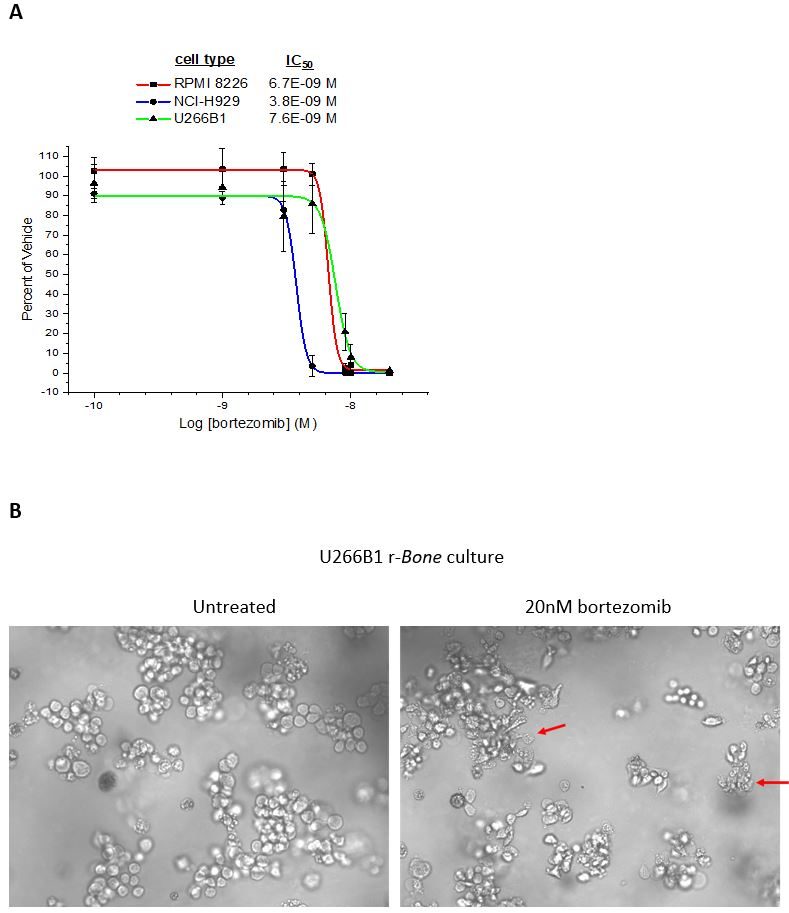
Figure 3: Bortezomib inhibition of myeloma cell proliferation in r-Bone culture
The r-Bone assay is a potential platform for studying multiple hematologic malignancies in vitro as well as the investigation of solid tumor metastasis, like breast and prostate carcinomas to the bone matrix.
Predictive Oncology® has provided customized tumor-specific 3D cell culture models since 2014 and has been actively developing numerous organ-specific matrices to broaden the preclinical tumor offerings since we've been working together.
Reconstructed mouse breast (r-mBreast) and human breast (r-Breast), lung (r-Lung), and prostate (r-Prostate), are currently being characterized and developed. These models will represent new early opportunities to screen test agents in a platform that will more closely mimic in vivo tissue microenvironments without investing in animal studies upfront. The potential to screen targeted chemical libraries in a high throughput 3D assay should be considered in early discovery efforts.
For more information on how these 3D model assays can be applied to your preclinical oncology and immuno-oncology research, contact our scientists below.
References
1. Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12(4):207–218.
Connect
Let's start a conversation
Contact Us