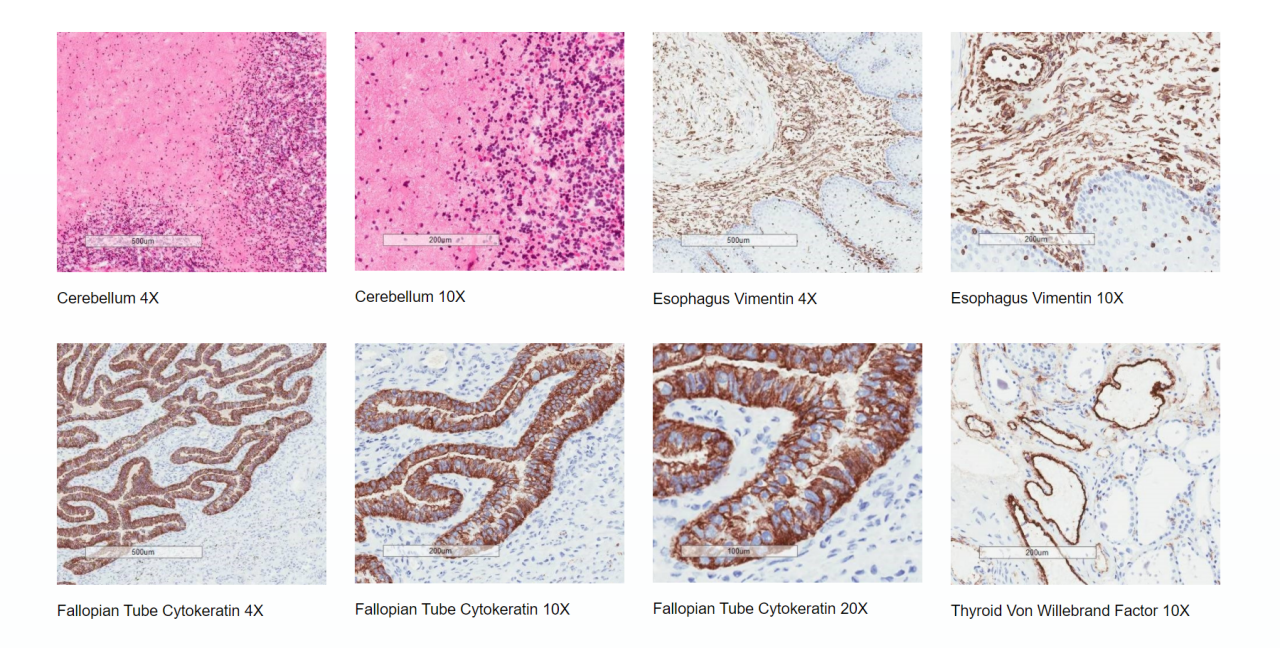
Tissue Cross Reactivity assessment of a therapeutic antibody or antibody-like molecule is an essential preclinical investigation that forms part of a regulatory Investigational New Drug (IND) or Clinical Trial Application (CTA).
TCR is a series of ex-vivo immunohistochemical (IHC) screening assays performed primarily to identify off-target binding, but also to potentially identify previously unknown sites of on-target binding.
The presence or absence of IHC staining in frozen tissues ex vivo may be used to indicate potential organ toxicity in vivo as well as providing additional justification for the choice of animal toxicity models used to generate pre-clinical safety data.